What does the electrostatic force do inside the nucleus. As we know that all subatomic particles that is neutrons and protons are bounded together by strong nuclear force inside the nucleus as the distance between them is very small.

Electrostatic Forces Are Applicable For Point And Static Charges But Electrons Are Continuously Revolving Around The Nucleus How Does It Exist In The Atom Quora
The electrostatic force acts over a distance of about one-tenth the diameter of an atomic nucleus or 10-16 m.

. The nuclear forces only have a short range while the electrostatic force doesnt. The word covalent describes the effect of two nuclei attracting a single electron and not the mechanism that attracts that electron. Since the Electrostatic force between protons is very small or negligible as we compare it with nuclear force so here we can say that if we add more number of protons in an atom then the.
But as the number of protons inside a nucleus increases the electrostatic repulsion between protons increase reducing the binding. 1 question What is an effect of electrostatic forces inside the nucleus. For example two positively charged protons repel each other as do two cations two negatively charged electrons or two anions.
The force on the electron is electric - and it is exerted by both nuclei in roughly the same proportions so there is an effective force keeping the two nuclei together despite their net positive charges. How the Electrostatic Force Works. Like charges repel one another while unlike charges attract one another.
Electrostatic force binds the negatively charged electrons to the positively charged atomic nucleus. What keeps electrons in the space around a nucleus. The nucleons are bound to each other by means of Strong Nuclear Force that is more than 100 times stronger than the electrostatic force.
The neutrons in the nucleus repel each other. Thus the electrostatic force determines the stability of a nuclear configuration. After sealing the tube the student mixes the solutions and notices a bubble that forms in the tube.
Astudent carefully transfers 30 g of water and 30 g of alcohol in a glass tube forming two layers and filling the tube completely. This is why when there are too many nucleons it also becomes radioactive. The protons and neutrons attract each other.
What is the mass of the contents in the glass tube after mixing. In an atomic nucleus you have a collection of both protons and neutrons and the nuclear force is at odds with the electrostatic force that wants to push the protons apart. The protons in the nucleus repel each other Explanation.
It causes the protons in the nucleus repel each other. Thus the electrostatic force determines the stability of a nuclear configuration. The number of protons inside a nucleus increases the electrostatic repulsion between protons increase reducing the binding energy.
The number of protons inside a nucleus increases the electrostatic repulsion between protons increase reducing the binding energy. The protons in the nucleus repel each other. As the nucleus of an atom contains only positively charged protons so in the nucleus of an atom the electrostatic force is of repulsive nature and hence causes repulsion between the various protons present in the nucleus.
The protons and neutrons repel each other.

What Is An Effect Of Electrostatic Forces Inside The Nucleus Brainly Com
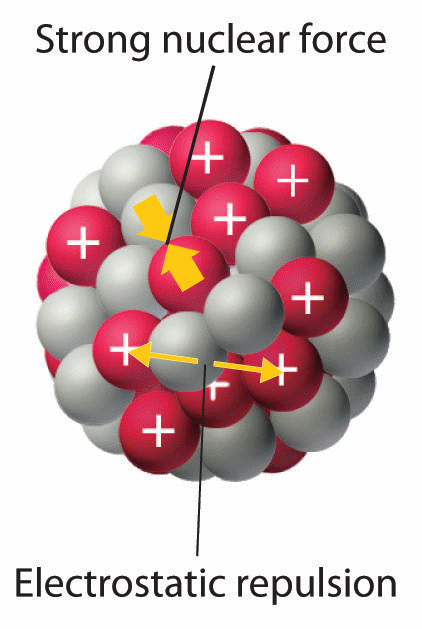
Mass Size And Electrostatic Forces Inside The Nucleus
Electrostatic Forces Are Applicable For Point And Static Charges But Electrons Are Continuously Revolving Around The Nucleus How Does It Exist In The Atom Quora
0 Comments